AIEgen-Based and Smartphone-Assisted On-Site Quantitation of Cystatin C for Monitoring of Chronic Kidney Disease
AIEgen-Based and Smartphone-Assisted On-Site Quantitation of Cystatin C for Monitoring of Chronic Kidney Disease
Xia, S. Q.#; Zhu, X.#; Niu, S. Q.#, Zhang, W. Q.; Gong, J. Y.; Luo, Z. F*.; Li, M. J.* Yi, C. Q*.
Aggregate, 2025
https://doi.org/10.1002/agt2.70127
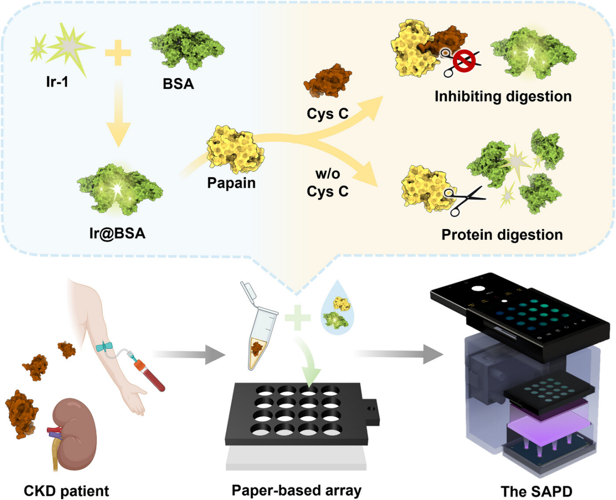
Abstract. The development of affordable and user-friendly diagnostic tools for early warning and monitoring progression of chronic kidney disease (CKD) is crucial to reducing CKD-related morbidity and mortality. This study reports on (1) a protein-templated AIEgen, Ir@BSA, which emits intense green phosphorescence with a quantum yield up to 69.40% and a lifetime up to 1839.40 ns in aqueous solution; (2) a straightforward protocol for Cys C quantitation, which employs Ir@BSA as the phosphorescent signal indicator and papain as the biomolecular recognition element, respectively; and (3) a smartphone-based portable phosphorescence reader (termed as SAPD), which can stably excite and accurately collect phosphorescence signals from the paper-based arrays. Quantitation of Cys C in clinical serum samples using SAPD integrated with the paper-based arrays highlights its remarkable advantages including high sensitivity (0.36 µg mL−1) and specificity, cost-effectiveness (∼$67.5 per set), portability (∼450 g), good precision (RSD ≤ 8.25 %), good accuracy (comparable to clinical standard latex immune-turbidimetric method), and high throughput (16 samples per experiment). More importantly, this study reveals the significant potential of Cys C as an early warning marker of CKD progression. The reported method enables Cys C quantitation anywhere, anytime, by anyone, and is ideally suited for mass screening for CKD and home monitoring of CKD progression, facilitating early diagnosis and proactive management of CKD.

